FLT3 status is key to AML patient care
Identifying patients who are FLT3 mutation-positive may provide both prognostic and selective value, due to the potential of tyrosine kinase inhibition.1 See below for more information.
FLT3 is selective
FLT3 positivity (ITD or TKD) is one of the eligibility criteria for treatment with RYDAPT.
At diagnosis
In parallel with cytogenetics. FLT3 test results are needed before day 8 of induction chemotherapy.
Every patient with newly diagnosed AML
All patients with newly diagnosed AML should receive a FLT3 test (except for those with acute promyelocytic leukaemia).
PCR followed by capillary electrophoresis
The method used during the RATIFY trial can analyse both mutations (ITD and TKD) in parallel.
FLT3 mutation testing to identify patients who are potentially eligible for RYDAPT is performed by The Laboratory of Personalized Molecular Medicine GmbH (Germany), a subsidiary of Invivoscribe Technologies, Inc.
Why test for FLT3?
Prompt FLT3 mutation testing at diagnosis now makes a difference. FLT3 testing significance has changed from prognostic in the past to selective today.
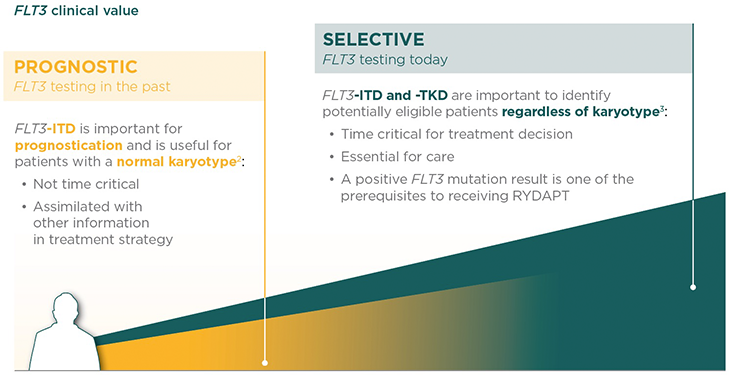
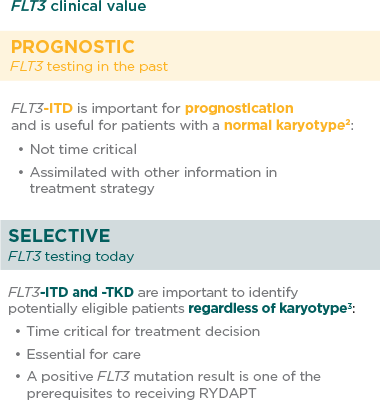
Who should be tested for FLT3?
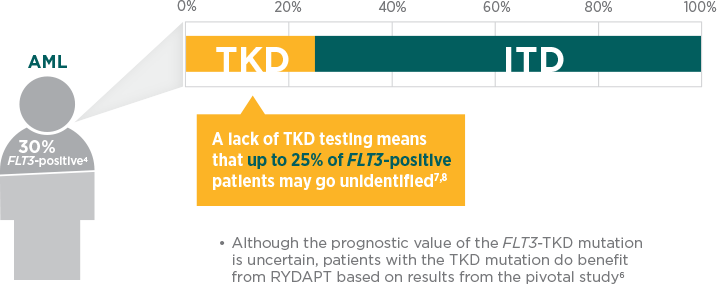
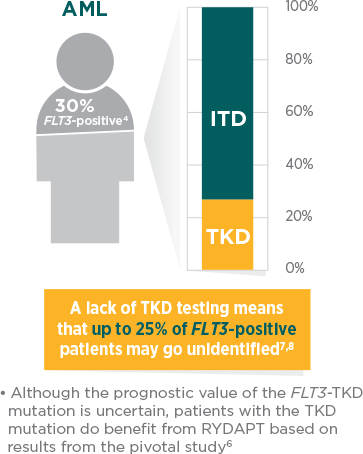
Diagnostic workup should include comprehensive testing for FLT3-ITD and -TKD mutations, which represent more than 30% of AML cases.1,4
Patients with ITD and TKD mutations were enrolled in the RATIFY trial and both types of patients benefited from RYDAPT.5,6
When should the FLT3 test be prescribed?
The European LeukemiaNet (ELN) 2017 recommendations advise testing at diagnosis and obtaining results within 3 days.1

FLT3 test turnaround time is important to support patient care in the acute setting.
According to the RATIFY phase 3 clinical trial, RYDAPT should be administered in sequential combination with induction chemotherapy starting on day 8 for patients with FLT3 mutations (ITD and/or TKD), irrespective of the cytogenetics findings.5
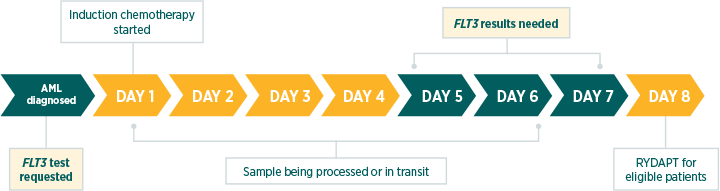
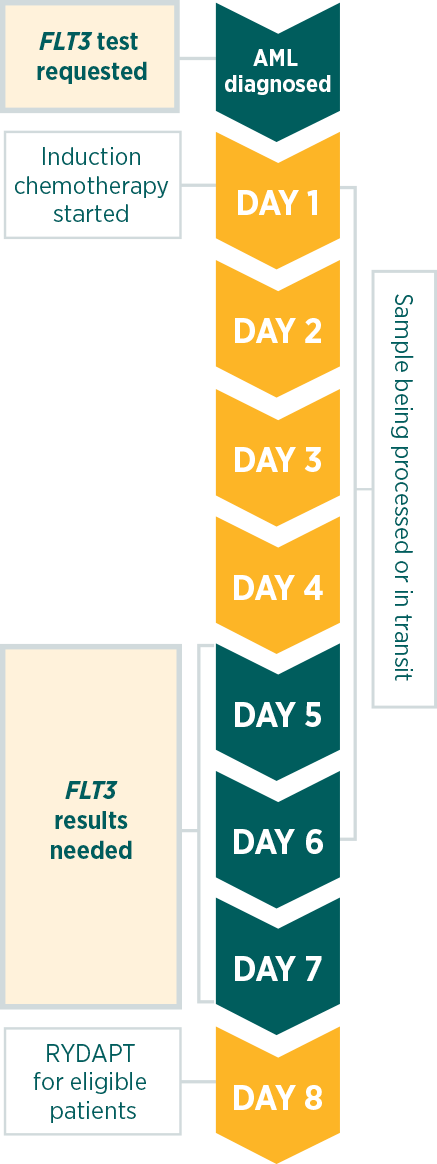
International guidelines recognise the clinical value of a FLT3 test at diagnosis as a selective marker. The latest ELN recommendations (2017) highlight the need for prompt, comprehensive FLT3 testing.1


How should the FLT3 test be performed?
Multiple methodologies are currently used for FLT3 testing in clinical practice.
The FLT3 test used in the RATIFY clinical trial was based on the Murphy et al and Thiede et al methods and analyses for both ITD and TKD mutations by capillary electrophoresis.9-11
This approach leads to a more efficient workflow in the lab compared to common methods and could ultimately lead to a faster determination of FLT3 status
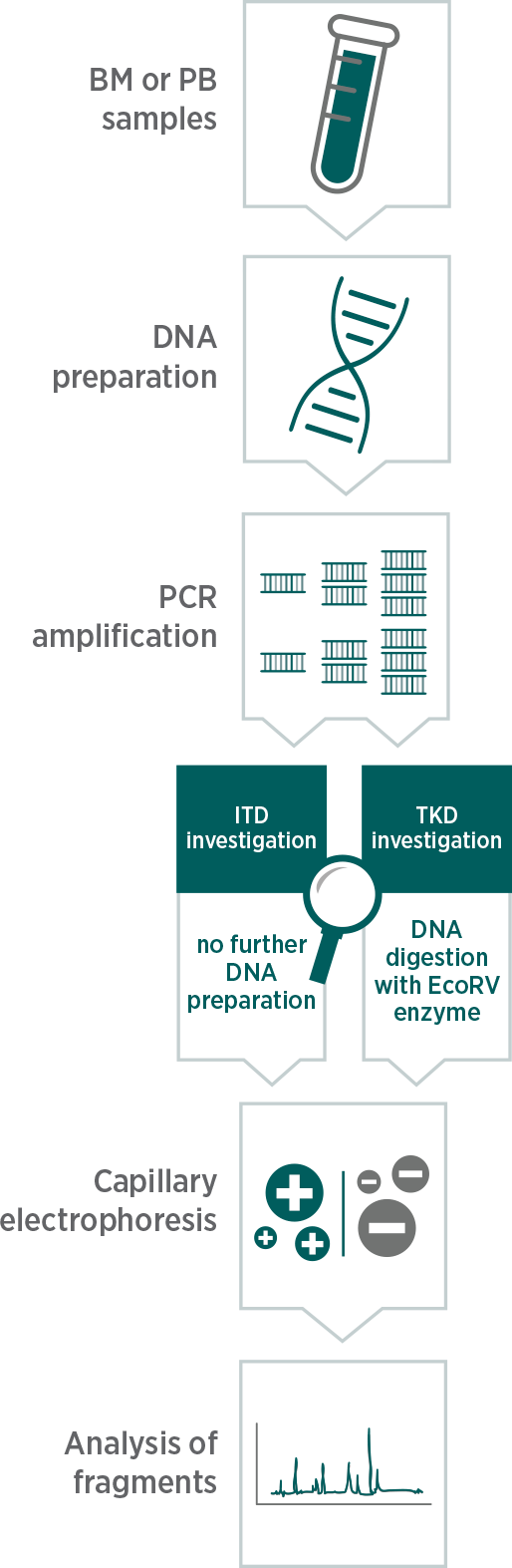
The RATIFY clinical trial assay considers that the FLT3 mutation is positive if the mean mutant-to-wild-type signal ratio meets or exceeds the clinical cutoff of 0.059
Patients with a signal ratio less than 0.05 are considered negative for the FLT3 mutation9
Download a brochure to learn more about FLT3 testing
AML, acute myeloid leukaemia; BM, bone marrow; FLT3, FMS-like tyrosine kinase 3; ITD, internal tandem duplication; PB, peripheral blood; PCR, polymerase chain reaction; TKD, tyrosine kinase domain.
References: 1. Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424-447. 2. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Acute Myeloid Leukemia V.2.2016. © National Comprehensive Cancer Network, Inc. 2016. All rights reserved. Accessed September 12, 2017. To view the most recent and complete version of the guideline, go online to NCCN.org. 3. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Acute Myeloid Leukemia V.3.2017. © National Comprehensive Cancer Network, Inc. 2017. All rights reserved. Accessed September 12, 2017. To view the most recent and complete version of the guideline, go online to NCCN.org. 4. Patel JP, Gönen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366(12):1079-1089. 5. RYDAPT [Summary of Product Characteristics]. Novartis Pharma AG; 2017. 6. Data on file. Study no. CPKC412A2301. Novartis Pharmaceuticals Corp; 2016. 7. Al-Mawali A, Gillis D, Lewis I. Characteristics and prognosis of adult acute myeloid leukemia with internal tandem duplication in the FLT3 gene. Oman Med J. 2013;28(6):432-440. 8. Santos FP, Jones D, Qiao W, et al. Prognostic value of FLT3 mutations among different cytogenetic subgroups in acute myeloid leukemia. Cancer. 2011;117(10):2145-2155. 9. Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation [supplementary appendix published online June 23, 2017]. N Engl J Med. 2017;377(5):454-464. 10. Murphy KM, Levis M, Hafez MJ, et al. Detection of FLT3 internal tandem duplication and D835 mutations by a multiplex polymerase chain reaction and capillary electrophoresis assay. J Mol Diagn. 2003;5(2):96-102. 11. Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99(12):4326-4335.






